
16 Oct 2023
One of the challenges we face is teams trying to figure out the right conditions for a reaction during scale-up from a process safety point of view. Especially for exothermic reactions this is a crucial problem since the risk of a runaway reaction is large. This is a very difficult problem yet a crucial one to get right. The process conditions can involve points such as:
Typically, teams are focused on yields and how to choose conditions to maximize conversion and selectivity. But a major element is process safety. If you choose conditions at the pilot scale there is very little chance to change them later during production. And this is often a crucial reason for an unsafe or sub-optimal plant.
A detailed discussion can be had with our process experts team at Amar info@amarequip.com however the purpose of this blog post is to educate our users about some of the vast literature that is available for considering process safety during scale-up.
What makes the problem especially difficult is the vast parameter space. With these process variables literally hundreds of combinations can be made and it is very difficult for a design of experiment to explore all of it.
A great reference on this topic is the book by Stoessel (Thermal Safety of Chemical Processes Risk Assessment and Process Design). We strongly advise our customers to refer to this encyclopedic reference for additional insights on the process safety of exothermic reactions.
The figure below (reproduced from the book above) shows five criticality classes identified by Stoessel based on the risk of a runaway reaction and the catastrophic results it may produce. In increasing order from Class-1 to Class-5 reactions become increasingly hazardous. There is rarely a good reason to work with a Class-5 reaction. Usually, process changes can be made to reduce the criticality class. Refer to Stoessel’s great book for additional details.
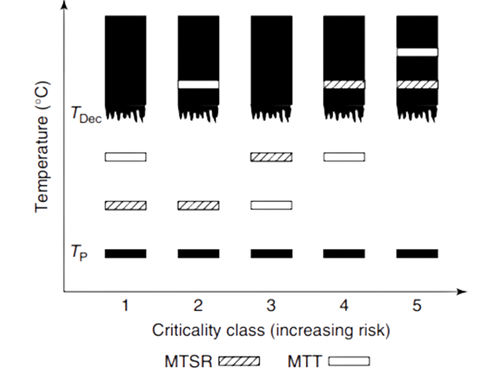
Legend for Figure above:
The message of this blog post is: Do not choose unsafe operating conditions and try to compensate by measures such as interlocks, PRVs, or RDs. It is wiser and cheaper to often modify process conditions to come up with an inherently safer reaction.
Stay tuned for more posts about process safety during design in the blog series. Meanwhile contact us for additional insights, info@amarequip.com. We will soon have a webinar on this topic and please add your email address to our mailing list to be notified. We promise we will not spam you! 😊