
21 Nov 2023
Process intensification (PI) refers to a strategy aimed at transforming chemical processes to make them significantly more efficient. This is achieved through the development of new equipment and production concepts, which can lead to substantial improvements in terms of process and energy efficiency, improved safety, and reduced costs. The key aspects of PI include the reduction of equipment size, the use of innovative process routes, the integration of multiple unit operations into single apparatuses, and the maximization of energy and material use.
One way to achieve higher throughput i.e. smaller reactors is via more aggressive Temperature conditions. The Arrhenius Law, formulated by Svante Arrhenius in 1889, is a fundamental principle that describes the temperature dependence of reaction rates. The law states that the rate of a chemical reaction increases exponentially with an increase in temperature. Mathematically described by the equation below:
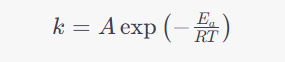
Where

As is obvious raising T usually will lead to higher reaction rates and hence smaller reactors.
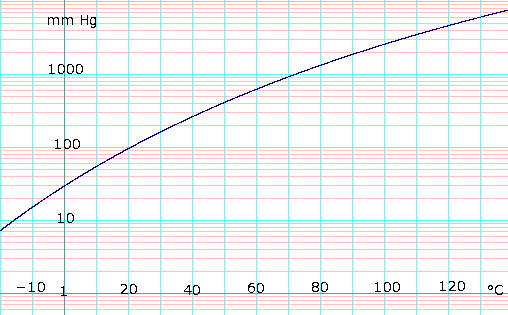
So far so good, but now the pesky problem is usually autogenous pressures. Or when solvents are involved the vapor pressures of solvents. Antoine's Law is an empirical equation that relates the vapor pressure of a pure substance to temperature. If you see the equation below, typically vapor pressure increases with temperature.

For example, the graph below (courtesy NIST / Wikipedia) shows the vapor pressure of methanol. If instead of atmospheric pressure you want to carry the reaction at 2x pressure the temperature can easily exceed 100 C (just the autogenous pressure, in reality, solubilities, activity coefficients, non-idealities, etc. will make the analysis more complex for a reaction)
The challenge is that during lab chemistries typical equipment is made of glass which has pressure limitations. Hence chemists usually run reactions at atmospheric conditions. If scale-up is performed with these T,P conditions, as is, plants end up with large reactors which are sub-optimal.
Enter high pressure reactors also called lab autoclaves. They allow reactions to be performed at conditions that lead to process intensification. Of course, you have to reconcile with selectivity, product degradation limits, etc. so this strategy has its limits. But in most projects, we do find that we have a lot of space for optimization.
We have a huge range of High Pressure autoclaves at Amar including equipment made from exotic materials like Hastealloy C.
The flow below illustrates the strategy:

The conclusion is: Think outside the box. Don't do reactions at atmospheric conditions just because the initial run in the lab used that condition. Buy a high pressure autoclave.
Please contact us for a consultation on your specific reaction.